Gilead Sciences, Inc. (Nasdaq: GILD) today announced the initiation of two Phase 3 clinical studies to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19 (novel coronavirus). These randomized, open-label, multicenter studies will enroll approximately 1,000 patients at medical centers primarily across Asian countries, as well as other countries globally with high numbers of diagnosed cases, beginning in March
Today is Mar 8, need two weeks for some prelim results. Have they started?
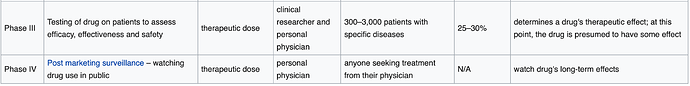
Noted only need 25-30% ![]() success rate to be considered effective - did I understand correctly?
success rate to be considered effective - did I understand correctly?